Chemicals help farmers and owners of small plots protect plants from diseases and increase soil fertility. In spring, crops especially need a macronutrient such as nitrogen, which is found in many industrial fertilizers. Ammonium nitrate has another name - ammonium nitrate, the product helps to increase productivity even in unfavorable climatic conditions.
Is it ammonium nitrate?
Ammonium nitrate is a pure nitrogen fertilizer in which the content of the main substance ranges from 26 to 34%. According to this indicator, ammonium nitrate is second only to urea. Manufacturers produce two types of chemicals: ammonium nitrate labeled “A” is intended for industrial use, and labeled “B” is intended for use in agriculture.
Ammonium Nitrate has another name - ammonium nitrate, and was first obtained in 1659 by the chemist Johann Glauber. Ammonium nitrate is used as a component of explosives and as a nitrogen fertilizer in agriculture. Its chemical formula is NH4NO3.
Domestic farmers and gardeners use nitrogen fertilizer to increase plant productivity and increase crop resistance to adverse weather conditions. It is used both as a single agent and in combination with other chemicals. Dry fertilizer is used in autumn and spring, and liquid solutions are suitable for root feeding in early summer.
Physical properties
Scientists, as a result of research on the substance, described its physical properties, which must be taken into account when applying fertilizers on their plots. The chemical is marketed in the form of a white or yellowish crystalline powder.
Solubility
The solubility of ammonium nitrate in water depends on the temperature of the liquid; So, at 0 degrees this figure is 119 g/100ml, and at 100 degrees - 1024 g/100ml. Ammonium nitrate is also soluble in methanol, ethanol and pyridine. When a substance decomposes, intense heat absorption occurs, which greatly slows down the dissolution process.
Compound
The composition of a chemical directly depends on its origin. In the first case, ammonium nitrate is obtained by neutralizing a nitric acid salt. In the second case, the substance is produced by chemical reactions of starting substances.
The main useful component of the fertilizer is nitrogen, it contains about 35%. In addition, ammonium nitrate contains 60% oxygen and 5% hydrogen. Despite the fact that plants need nitrogen to grow green mass and fully bear fruit, excessive application of fertilizer worsens the frost resistance of perennial crops and slows down the autumn growth of trees and shrubs.
The high nitrogen content in the fertilizer helps improve the process of photosynthesis, increase productivity and extend the shelf life of fruits.
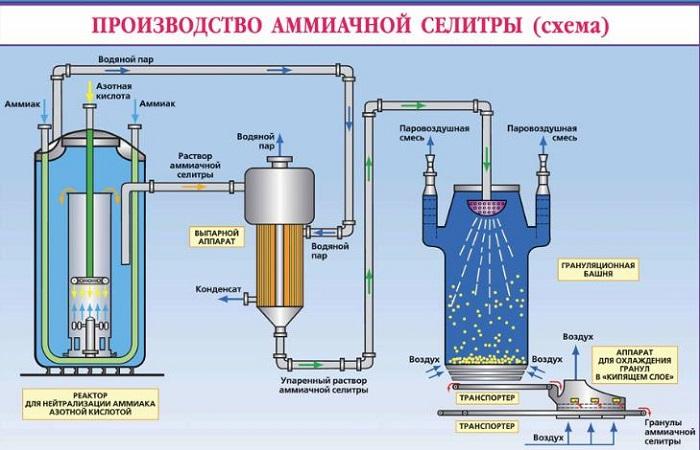
Receipt methods
The principle of ammonium nitrate production is based on the neutralization of nitric acid with ammonia gas. After this, the resulting solution is evaporated to form crystals or powder. To extend the shelf life of the fertilizer, it is coated with special compounds that prevent caking.
The ammonium nitrate production technology consists of the following stages:
- The process of neutralizing nitric acid with ammonia.
- Evaporate the resulting solution.
- Salt crystallization process.
- Drying and cooling a substance.
Since the process is accompanied by intense heat generation, carrying out the procedure outside the factory environment is quite dangerous.
Chemical properties
When exposed to high temperatures, ammonium nitrate decomposes. This process triggers the release of a large amount of heat and oxidizing agent. Ammonium nitrate also reacts with alkaline substances, during which ammonia is released. Caution must be exercised in chemical processes as the substance is explosive.
Instructions for use as fertilizer
Due to the high nitrogen content in the fertilizer, it is recommended to use it in the following cases:
- for feeding ornamental plants and flowers (promotes intensive growth of green mass);
- for saturating any type of soil with nitrogen;
- for feeding cultivated plants at any stage of the growing season.
Since saltpeter is explosive, all work with fertilizer must be carried out with extreme caution and follow the manufacturer’s recommendations.
When to apply to the soil
Ammonium nitrate is applied both in spring and autumn. If the soil on the site is light, it is recommended to do this before sowing cultivated plants. In heavy and clayey soil, fertilizer will need to be used both in spring and autumn. The bulk of ammonium nitrate is added to the soil during the spring months and early summer to stimulate the development and growth of green mass of crops.
As for dosages, on poor soils the consumption rate is 35 grams of fertilizer per square meter. If the soil is constantly fed with organic matter and mineral fertilizers, then 25 grams for the same area will be enough.
Application rates for different plants
The permissible amount of fertilizer depends on the type of crop that is grown on the site.It is allowed to use mineral fertilizer not only for adult plants, but also for seedlings in order to strengthen them and increase their stability after planting in open ground. Pour a tablespoon of ammonium nitrate under each bush.
Consumption rates for adult crops are as follows:
- Vegetable plants. Fertilizer is used twice during the growing season of crops - before the buds appear and at the time of fruit set. Each time take from 5 to 10 grams per square meter of garden.
- Fruit and berry trees. The first time fertilizing is applied in dry form in the spring, when leaves begin to appear on the crops, maintaining a dosage of 15 to 20 grams of fertilizer per square of garden. The second and third time, a liquid form of the chemical is used to feed the plants. 30 grams of powder or granules are diluted in 10 liters of clean water and this solution is used for watering the roots.
- Root crops. To apply fertilizer between rows of plantings, make shallow furrows and sprinkle ammonium nitrate into them at the rate of 7 grams of fertilizer per square meter. Fertilizer is applied once per season, three weeks after plant germination.
- Flower crops. The plants are fed with a liquid form of fertilizer. 10 granules of ammonium nitrate are dissolved in 1 liter of water and root feeding is carried out.
Fertilizing winter crops
Plants planted before winter are fertilized with ammonium nitrate in the fall. 300 kg of fertilizer in the form of powder or granules is consumed per hectare of field.
Application against weeds
In order to destroy weeds on the field, you will have to make a concentrated solution. To do this, dissolve 3 kg of ammonium nitrate in a bucket of water. Weeds are sprayed with this liquid.The solution destroys all types of weeds, but does not harm the soil or human health, since the ammonia evaporates after some time.
Security measures
When working with chemicals, follow safety rules so as not to harm your health. Be sure to use clothing that covers all parts of the body, gloves and a respirator so that ammonium nitrate vapors do not penetrate the respiratory tract.
Advantages and disadvantages
Like any fertilizer, ammonium nitrate has its advantages and disadvantages, which must be studied before use.
The advantages of the chemical include:
- low cost compared to other mineral supplements;
- no need for deep embedding;
- double action - both short-term and prolonged;
The disadvantages of the drug include:
- risk of spontaneous combustion and explosion if used incorrectly;
- loss of performance when stored in a damp room.
Storage rules
Keep the chemical in a dry and dark utility room.