Nitrogen fertilizers, such as nitrate, have long been successfully used in agriculture to feed plants. Let's consider the physical and chemical properties of sodium nitrate or sodium nitrate, benefits and harms, use, shelf life and storage rules, and contraindications for use. How to prepare saltpeter yourself, how to act in case of poisoning.
What is sodium nitrate
Sodium nitrate belongs to nitrogen fertilizers, containing 15-16% nitrogen and 26% sodium.In appearance it is a white salt consisting of small crystals. Saltpeter dissolves well in water, so that it dissolves faster, it is advisable to use warm rather than cold water.
Physical properties
Sodium nitrate is not the only name for the substance. It is also called sodium nitrate or Chilean saltpeter (since the large deposit where salt was first mined was located in Chile). Currently, salt is not only mined in different countries, but is mainly obtained as a by-product that is formed during the production of nitric acid from ammonia.
The fertilizer is soluble in plain water, solubility increases with increasing temperature, at 20 °C 87.6 g of powder dissolves in 100 g of water, at 60 °C – 124.7 g, at 100 °C – 176 g.
Chemical properties
Sodium nitrate has the formula NaNO3. Absorbs moisture from the air, as a result of which intensive caking occurs, a structural change occurs, recrystallization of small crystals into large ones. In a dry place it does not cake, remains crumbly, and in this form it is convenient to apply it to the soil.
In soil, sodium nitrate breaks down into the Na+ cation and the NO3– anion. Nitrate ion is absorbed only biologically; in autumn and winter, due to the lack of biological absorption, it does not remain in the soil. Because of this, nitrogen losses occur, especially on light-textured soils. Because of this feature, sodium nitrate is not recommended for autumn application. If pre-winter application is necessary, the dosage should be increased.
Benefits and harms
Advantages of sodium nitrate:
- nourishes plants with 2 important elements: nitrogen and sodium;
- nitrogen in nitrate form, most accessible to plants;
- alkalizes the soil;
- can be used as a fertilizer for sowing, planting, and for feeding;
- approved for use for all crops.
Disadvantages: sodium nitrate is not used for the main application in the fall, as it is easily washed out and does not bring any benefit.
Area of use
Sodium nitrate can be used on all types of soil, with the exception of solonetzes, since sodium contributes to even greater soil salinity. Neutralizes high acidity on sod-podzolic and light soils. For such soils, sodium nitrate is more effective than fertilizers with acidic ammonia.
For the main application, sodium nitrate is used in April, for pre-sowing (as a row fertilizer when sowing seeds) - in the next month, for root and foliar feeding - from June to August. Sodium nitrate is especially recommended for feeding root vegetables and tomatoes, as crops that love sodium. When feeding beets, it makes them sweet due to the increased outflow of carbohydrates from the foliage into the root crops. It also becomes a source of nitrogen for crops throughout the growing season.
How to make sodium nitrate yourself
You can prepare sodium nitrate with your own hands. To create fertilizer, 2 reagents are needed: ammonium nitrate and potassium chloride. They need to be taken in equal proportions. Separately, dissolve each component of the future mixture in water: 1 part of saltpeter in 3 parts of water and 1 part of potassium in 2 parts of water. Mix until dissolved and combine both components. After this, put it on fire.
Ammonia fumes will begin to be released, which are toxic if inhaled, so you need to prepare saltpeter outdoors or in a well-ventilated area.When the bubbles and evaporation stop, cool the solution and put it in the refrigerator. The saltpeter is ready when long white crystals form. They need to be crushed to a powdery state. Place in paper bags or thick plastic bags. Store in a dry place.
Instructions for use
Sodium nitrate is applied to all crops, except those that do not require a lot of sodium as a nutrient. The dosage of fertilizer in agriculture depends on the amount of nitrogen in the soil, weather and climatic conditions, the type of crop being grown and the previous one. The rate of use of sodium nitrate is not constant; it must be calculated in each case separately.
Approximate dosage for main application: for root crops – 50 g per 1 m², for vegetables – 40 g per 1 m²; for flowers – 35-40 g per 1 m². To prepare liquid fertilizing solutions, 100 g of saltpeter is dissolved in 10 liters of water, and this volume is consumed per 10 m².
When used in private household plots, saltpeter is applied during digging in the fall, 1-2 kg per hundred square meters, and 0.5 kg in the spring. When planting trees, pour 100-150 g of fertilizer into each planting hole, and 60 g of fertilizer under a bush. You can also sprinkle the powder in tree trunk circles - 15-30 g per 1 square meter. m; for an adult tree, the maximum dosage is no more than 250 g per plant.
For ease of dosage, you can use improvised means: 1 tsp. holds 5 g of fertilizer in 1 tbsp. l. – 15 g, in a matchbox – 25 g.
Contraindications and storage rules
It is not recommended to use on salt marshes and saline soils. For them you need to choose some other fertilizer with a similar effect. When used together, it should not be mixed with humus or additives including phosphorus and potassium. Not used in greenhouses.
The shelf life of sodium nitrate is unlimited. Store only in original packaging that does not allow moisture to enter into the powder. Do not store next to organic fertilizers, food, or household products. Keep in a warehouse inaccessible to animals and children.
When heating the fertilizer, there is a risk of fire or explosion, so it is isolated from flammable materials and hermetically sealed. Bags of powder are stacked on pallets.
Safety precautions and actions in case of poisoning
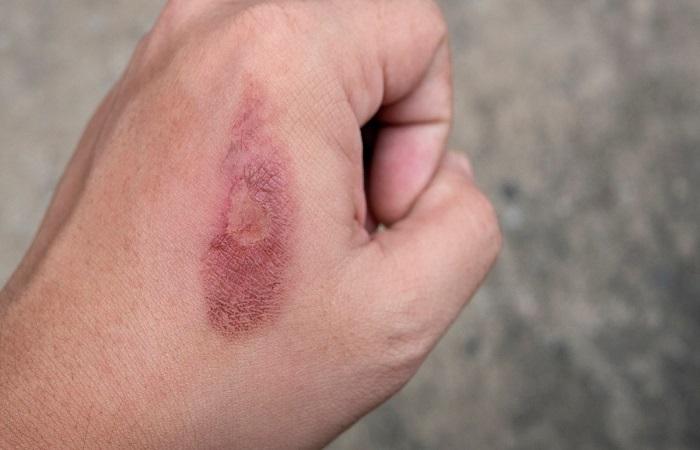
Sodium nitrate in contact with skin has an irritating effect on it. To avoid this, wear rubber gloves when working with powder. When the work is finished, wash your hands. It is also necessary to wear a respirator and goggles; a respirator is especially necessary when preparing sodium nitrate yourself.
Sodium nitrate is toxic to humans and can be dangerous if inhaled or if it comes into contact with mucous membranes or skin. Dust is irritating and can cause chemical burns.
Sodium nitrate poisoning can occur if safety rules are violated or the dosage is exceeded. In this case, the following symptoms are observed: a salty and bitter taste, nausea, convulsions, lethargy, pain in the liver area and in the back of the head, cyanosis.
If you experience symptoms indicating serious poisoning, you should immediately seek help from a doctor.Be in the fresh air, warm, change clothes. Rinse skin with water and eyes with cold water for 10-30 minutes. If the solution gets into the stomach, you need to rinse: drink 6-7 pcs. activated carbon tablets, wash down with water. When 20 minutes have passed, artificially induce vomiting.
Sodium nitrate is one of the nitrogen fertilizers, used to feed all crops, but is especially recommended for application to root crops and nightshade crops - tomatoes, potatoes. It contains nitrogen in a nitrate form that is easily digestible by plants. Can be used to alkalize acidic soils. It is not recommended for autumn application on light and flooded soils due to leaching into the lower layers of the soil.